Abstract
Venetoclax is the first BCL-2 inhibitor approved for the treatment of patients with chronic lymphocytic leukemia (CLL) alone or in combination with anti-CD20 monoclonal antibodies (MoAbs). Initial studies have shown high efficacy of venetoclax monotherapy or in combination with rituximab with an overall response rate of 79-92% in patients with relapsed/refractory (R/R) CLL.
To investigate the use of venetoclax combinations in a real-world population, we designed this observational retrospective and prospective study aimed at defining the outcome of patients with R/R CLL treated with venetoclax-based regimens outside clinical trials in Italy. Efficacy (progression-free survival -PFS-, overall response rate -ORR-, complete response -CR-, measurable residual disease -MRD-) and safety (most frequent adverse events -AE-, grade 3-4 AE, laboratory and clinical tumor lysis syndrome -TLS-) data were collected within the study through a web-based platform after patients signed written informed consent.
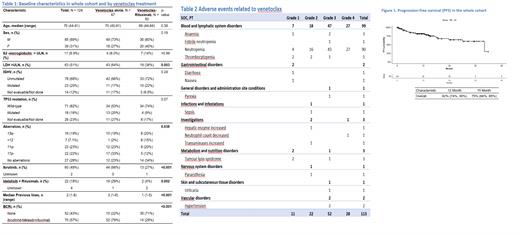
As of July 8 th, 2021 124 subjects (85 males, 39 females) were enrolled into the study (Table 1). Median age at venetoclax initiation was 70 years (44-91), median CIRS score 4 (0-14), median creatinine clearance 70 ml/min (22-123). A relevant proportion of patients presented adverse prognostic features, including TP53 aberrations [32% TP53 mutation and/or del(17p)], unmutated IGHV (77%). At the time of venetoclax initiation, patients had received a median of 2 previous therapies (1-8), with 57% previously exposed to BTK and/or PI3K inhibitors. 67/117 (57%) patients received venetoclax alone, while 50 were treated with a combination of venetoclax + rituximab (VenR), information on treatment plan was missing in 7 patients. Patients on venetoclax monotherapy compared to those receiving VenR showed a higher percentage of elevated LDH at baseline (64% vs 38%), were more heavily pretreated (median lines of therapy 3 vs 1), and had more frequently received treatment with ibrutinib (66% vs 27%) and/or idelalisib + rituximab (29% vs 4%). After a median follow-up of 13.7 months (0-41.9), the estimated 12m-PFS was 82% (CI 74-90%) (Figure 1), and the estimated 12m-overall survival (OS) was 83% (CI 76-91%). The best ORR in the whole cohort was 85% (CI 95% 76-91%) with a CR rate of 40%; best response was reached after a median of 3.9 months of treatment (range 0.6-30.5). The best ORR was not different in patients receiving VenR vs venetoclax alone (83% vs 88% respectively, p = n.s.), while the CR rate was significantly higher in those receiving VenR vs venetoclax alone (57% vs 30%, p=0.011). The ORR and CR rate in patients previously exposed to BTK and/or PI3K inhibitors were significantly lower (77% and 29% respectively), and the 12m-PFS was shorter in patients previously treated with ibrutinib compared to ibrutinib-naïve (75% vs 89%, p=0.005). Twenty subjects discontinued venetoclax (median time to discontinuation 4.1 months, range 0.4-10.8), 8 due to disease progression, 3 Richter's transformation, 7 AEs, 1 was lost to follow-up and 1 underwent planned allogeneic stem cell transplantation.
Venetoclax-based regimens were well tolerated, with the most frequent AE of any grade related to venetoclax being neutropenia (79.6%), grade 3-4 in 61.9% (Table 2). In the whole cohort one grade 3 clinical TLS occurred and resolved without sequelae, and only 2/124 patients experienced laboratory TLS during ramp-up phase (both grade 1). No treatment-related death was reported. In conclusion, this analysis presents the results of one of the largest cohort of patients with R/R CLL treated with venetoclax-based regimens outside clinical trials and confirms the favourable efficacy and safety profile of the drug. In this patient population highly enriched for unfavorable disease features (1 out of 3 carrying TP53 aberrations, almost 80% with unmutated IGHV) venetoclax-based regimens were able to obtain a response in a high proportion of cases, with a very short time to best response (less than 4 months). As expected, response duration was shorter in patients previously exposed to BTKi/PI3Ki. When we compared the outcome of patients treated with venetoclax monotherapy vs VenR, the addition of anti-CD20 MoAb allowed to reach deeper responses by significantly increasing the CR rate. As the study and its data collection are still ongoing, the updated analysis of an expanded cohort with longer follow-up will be presented at the meeting.
Scarfo: Janssen: Honoraria, Other: Travel grants; Astra Zeneca: Honoraria; Abbvie: Honoraria. Quaglia: Roche: Other: Travel grants; Janssen: Honoraria, Other: Travel grants, Speakers Bureau; Sandoz: Consultancy; AstraZeneca: Honoraria. Marasca: AbbVie: Honoraria, Other: Travel grants; AstraZeneca: Honoraria; Janssen: Honoraria, Other: Travel grants. Sanna: Astra Zeneca: Consultancy; Abbvie: Consultancy; Janssen: Consultancy. Murru: Abbvie: Consultancy, Honoraria, Other: travel and accommodation; Janssen: Consultancy, Honoraria. Laurenti: Roche: Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Gilead: Honoraria; BeiGene: Honoraria. Gaidano: Incyte: Membership on an entity's Board of Directors or advisory committees; Beigene: Membership on an entity's Board of Directors or advisory committees; Astrazeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Reda: Beigene: Consultancy; Astra Zeneca: Consultancy; Abbvie: Consultancy; Janssen: Consultancy. Molica: Astrazeneca: Honoraria; Abbvie: Consultancy, Honoraria; Janssen: Consultancy, Honoraria. Sportoletti: AbbVie: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria. Galimberti: AbbVie, Janssen: Honoraria, Other: Travel grants; Incyte: Speakers Bureau. Mauro: AbbVie: Consultancy, Speakers Bureau; AstraZeneca: Consultancy, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; Roche: Consultancy, Speakers Bureau; Gilead: Consultancy, Research Funding, Speakers Bureau; Takeda: Consultancy, Speakers Bureau. Cuneo: Janssen: Consultancy, Speakers Bureau; AstraZeneca: Consultancy, Speakers Bureau; AbbVie: Consultancy, Speakers Bureau; Gilead: Consultancy, Speakers Bureau. Ghia: AstraZeneca: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Acerta/AstraZeneca: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Research Funding; BeiGene: Consultancy, Honoraria; Celgene/Juno/BMS: Consultancy, Honoraria; ArQule/MSD: Consultancy, Honoraria; Sunesis: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal